Voris, Marshall D. An investigation of the effectiveness of cranial electrotherapy stimulation in the treatment of anxiety disorders among outpatient psychiatric patients, impulse control parolees and pedophiles. Delos Mind/Body Institute, Dallas and Corpus Cristi, Texas, 1995.
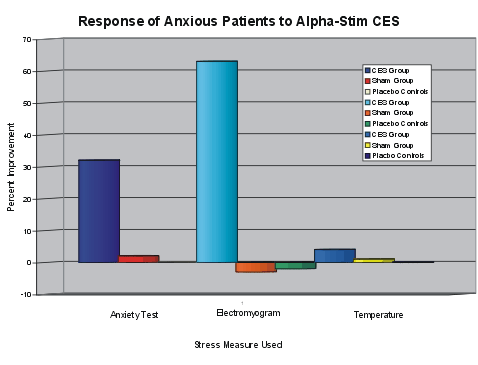
After achieving Institutional Review Board approval and informed consent, 105 parolees and psychiatric patients diagnosed with anxiety (STAI>40, EMG>6ÁV, Finger Temperature 75 - 95) were randomly assigned to either group A: Alpha-Stim CES (N = 40), group B: sham CES (N = 35), or group C, placebo controls (N = 30) by assigning conditions to chairs, during group sessions conducted over a 10 day period, and allowing the subjects to sit wherever they would like in a group setting. The study was triple-blind which was accomplished by the use of identical double-blinding boxes, with either of 2 options selected, and by the statistician being blinded to all conditions for analyses. All subjects were tested on the State Trait Anxiety Inventory (STAI), electromyogram (EMG), and finger temperature before and after a single 20 minute application of CES treatment, except the placebo control group, which sat in the same room but was not connected to any device. All CES subjects, but not sham treatment or placebo controls had significant effects. Analyses was by group medians. STAI in the active CES group had a pretest median of 50 ▒ 8.12 to post test median of 34 ▒ 7.39. STAI in the sham group had a pretest median of 51 ▒ 8.58 to post test median of 50 ▒ 8.46. STAI in the placebo control group had a pretest median of 48 ▒ 5.96 to post test median of 48 ▒ 8.81. EMG in the active CES group had a pretest median of 10.40 ▒ 7.12 ÁV to post test median of 3.80 ▒ 4.98 ÁV. EMG in the sham group had a pretest median of 12.55 ▒ 10.45 ÁV to post test median of 12.95 ▒ 10.65 ÁV. EMG in the control group had a pretest median of 8.00 ▒ 9.11 ÁV to post test median of 9.95 ▒ 9.69 ÁV. Temperature in the active CES group had a pretest median of 90.0║ F ▒ 5.63 to post test median of 93.60║ F ▒ 3.36. Temperature in the sham group had a pretest median of 90.5║ F ▒ 4.90 to post test median of 91.65║ F ▒ 4.81. Temperature in the placebo control group had a pretest median of 92.2║ F ▒ 5.59 to post test median of 92.45║ F ▒ 5.89. The probability of achieving these results by chance on the STAI is P<.0001 for CES vs. sham or controls, and P<.3902 for sham vs. controls, on the EMG is P<.0001 for CES vs. sham or controls, and P<.6693 for sham vs. controls, and on peripheral temperature is P<.0141 for CES vs. sham, P<.0011 for CES vs. controls, and P<.3109 for sham vs. controls.
The author stated that when they began the research, several members of the team had significant doubts as to the effectiveness of this technology. In addition, the concerns of FDA caused further concern and suspicion regarding this technology. But after this project, they concluded that they are completely convinced as to the value of this treatment methodology for many of their patients. No negative side effects were reported.
Note: The FDA visited this researcher 4 times but could find no problems with this research.
|