Lichtbroun, Alan S., Raicer, Mei-Ming C., and Smith, Ray B. The treatment of fibromyalgia with cranial electrotherapy stimulation. Journal of Clinical Rheumatology, 7(2):72-78, 2001. Presented at the Fifteenth Annual International Symposium on Acupuncture and Electro-Therapeutics, Columbia University, New York, October 1999.
After successful clinical use of Alpha-Stim cranial electrotherapy stimulation (CES) in the first authorís rheumatology practice, and IRB approval from Robert Wood Johnson Medical School, a double-blind, placebo-controlled study was undertaken in which 60 randomly assigned patients who completed informed consent were given either 3 weeks of subsensation (100 microamperes) CES treatment at 0.5 Hz for one hour daily (N=20), sham treatment (N=20), or served as controls for any placebo effect in the sham treated patients (N=20). All patients met the diagnostic criteria set forth by the American College of Rheumatology. The age range was from 23 to 82 years (mean of 50). There were 2 men and 58 women suffering from fibromyalgia from 1 to 40 years, (mean of 11 years).
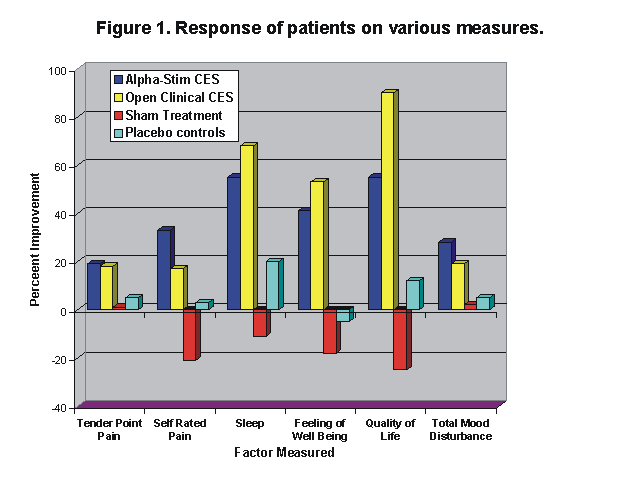
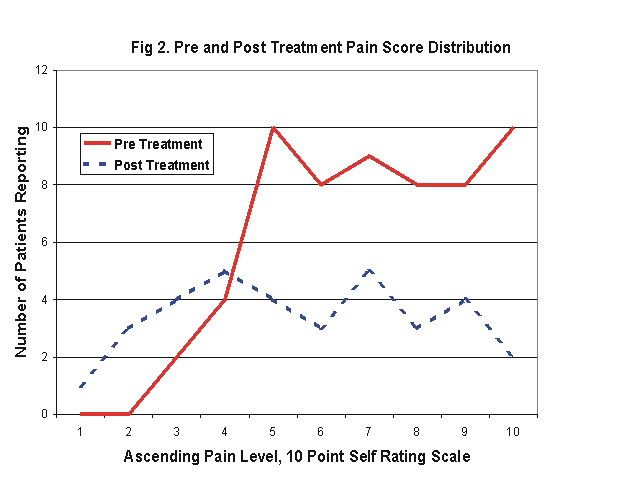
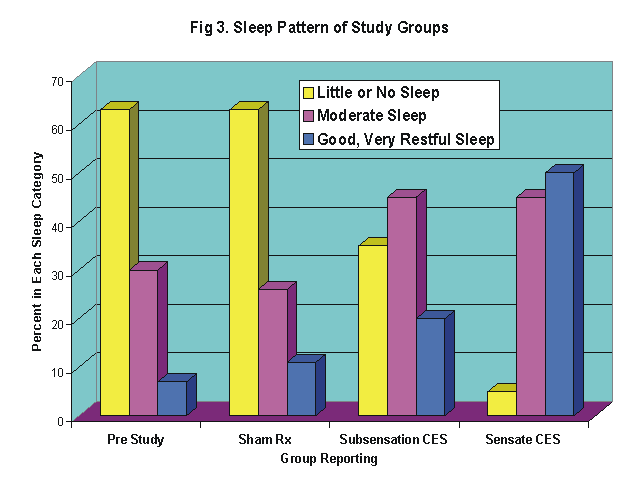
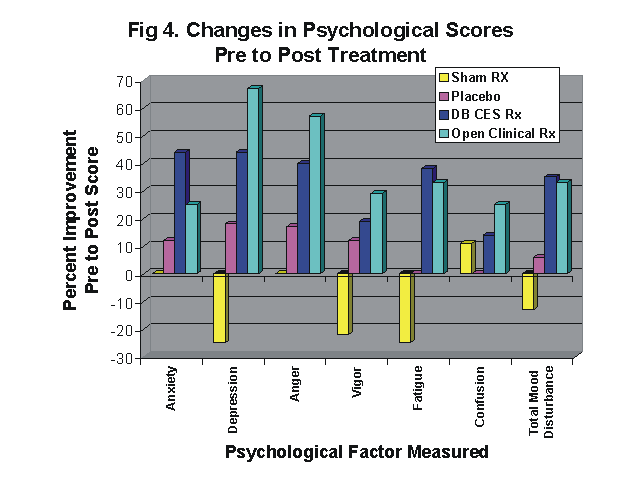
Treated patients showed a significant improvement in tender point scores (p<.01), and a significant improvement in self-rated scores of general pain level (p<.002). The number of subjects rating their quality of sleep as poor dropped from 60% at the beginning of the study to 5% (p<.02). In addition, there were significant gains in the self-rated feeling of well-being (p<.05), and quality-of-life (p<.03), plus fairly dramatic gains in six stress related psychological test measures of the Profile of Mood States. No placebo effect was found among the sham treated patients.
After the double-blind study, 23 of the 40 control patients opted for actual CES in an open clinical trial where they could increase the current in accordance with the standard clinical protocols for Alpha-Stim CES. They also showed a significant improvement in tender point scores (p<.001), and in self-rated pain (p<.005), quality of sleep (p<.001), feeling of well-being (p<.001), and quality-of-life (p<.001). Overall there was a 27% reduction in self-rated pain, and a 28% decrease in the tender point scores of the treated group.
According to a review of 34 studies of drug treatment for fibromyalgia, adverse effects from drugs are seen in 20% of fibromyalgia patients who use them, and improvement from prescription drugs was reported as 28% at best. In addition, unlike with the use of medication, there is no ongoing cost to the patient after purchase of the CES device. Accordingly, the authors concluded that Alpha-Stim CES is as effective as the drug therapies, with no negative side effects, and deserves further consideration as an additional agent for the treatment of fibromyalgia.